18+ Polar Or Nonpolar Calculator
Web Determine Chemical Electro-negativity and Bond Polarity. If its less than 04 the bond is nonpolar.

Solution 34507 Performing Polar And Rectangular Conversions On The Ti 83 Plus And Ti 84 Plus Family Of Graphing Calculators
Web Calculate the molecular polarity polar non-polar of a bond based on the electronegativity of the elements using this online chemical bond polarity calculator.

. Web In the video on electronegativity we learned how to determine whether a covalent bond is polar or nonpolar. Distinguish between the following three types of intermolecular forces. You can also use our tool as an electronegativity difference calculator to determine the difference between the electronegativity values of elements.
Web To determine if a molecule is polar or nonpolar it is frequently useful to look at Lewis structures. The calculator will then indicate whether the molecule is polar or nonpolar based on its calculated dipole moment. The calculator will analyze the molecules structure and electron distribution to determine its dipole moment.
Chemical bond polarity is the concept that explains the property of sharing an electron between two elements. In this video were going to see how we figure out whether molecules are polar or nonpolar and also how to apply that polarity to what we call intermolecular forces. Covalent bond between the elements can be either polar or non-polar.
Web Differences in electronegativity between two atoms can be used to determine if their bond is nonpolar polar or ionic. Nonpolar covalent bonds have an equal distribution of electron density between the two nuclei. Web To determine whether or not the molecule is polar you have to look at the partial charge vectors on the two bonds in the molecule.
First there are two electron pairs on the molecule which means there will be a large negative partial charge vector in that direction. Web Learn to determine if a molecule is polar or nonpolar based on the polarity between bonds and the molecular geometry shape. Nonpolar compounds will be symmetric meaning all of the sides around the central atom are identical - bonded to the same element with no.
Web Polar substances tend to dissolve in polar solvents and nonpolar substances dissolve in nonpolar solvents. Web The electronegativity calculator allows you to calculate the type of bond formed between different elements using their electronegativity values. Web Compute Dipole Moment.
Next oxygen is more electronegative than hydrogen and will hog the electrons. If the electronegativity difference is greater than 04 the bond is generally considered polar. This is determined with the concept of electro-negativity.
By comparing the Electronegativity of the two atoms one can determine if the. Dipole-dipole forces London dispersion forces and hydrogen bonds. Web Describe how molecular geometry plays a role in determining whether a molecule is polar or nonpolar.
Web The bond polarity between two atoms can be estimated if you know the electronegativity of both elements. Web You can calculate whether a bond is polar or not by finding the electronegativity difference between the two atoms involved. Web This electronegativity is rated on a scale known as the Pauling scale and if a molecule has an electronegativity between 0500-200 then it is considered polar and if it is less than 050 it is considered nonpolar.
Nonpolar bonds have no. We start with the polarity between bonds using the electronegativity. Web Molecule Polarity - PhET Interactive Simulations.
Web To determine if a molecule is polar or nonpolar it is frequently useful to look at Lewis structures. When a solute dissolves in a solvent the individual particles of the solute separate from their neighbours and move between the spaces of the solvent particles. Calculate the electronegativity difference ΔEN and average EN of the two electronegativities and use the table below to determine the bond type and polarity.
Nonpolar compounds will be symmetric meaning all of the sides around the central atom are identical - bonded to the same element with no. When two atoms bond to form a molecule the electron s in the bond are not necessarily shared equally. Polar covalent bonds have an unequal distribution of electron density with the more electronegative atom having greater electron.
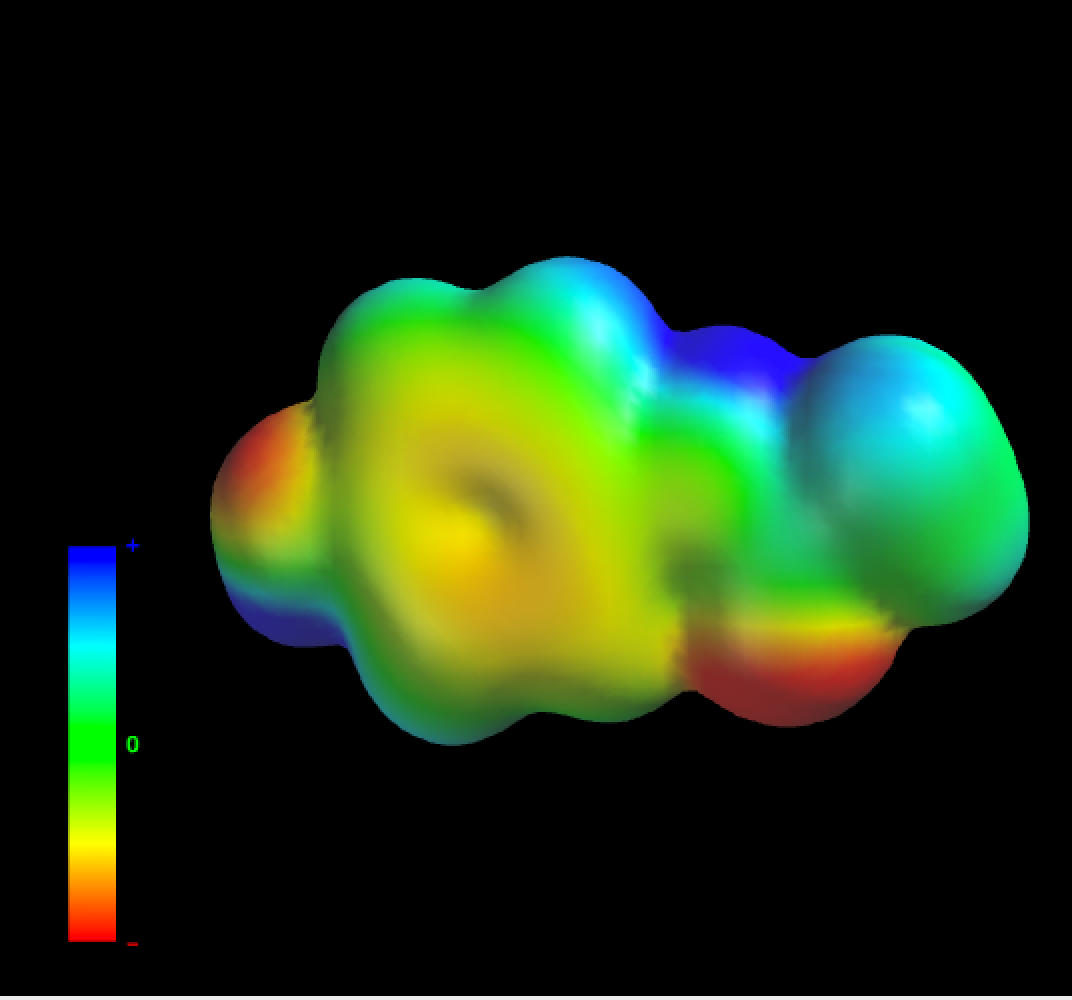
Polar Surface Area Wikipedia
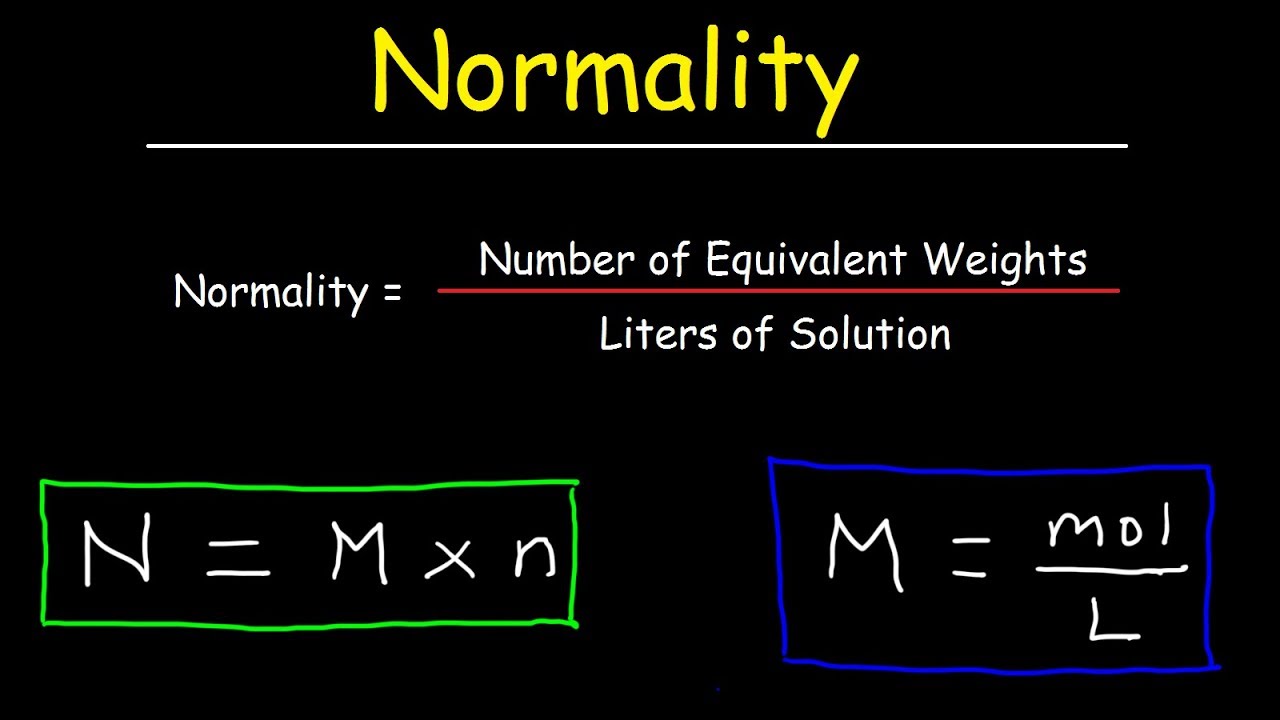
How To Calculate Normality Equivalent Weight For Acid Base Reactions In Chemistry Youtube
Is C3o3h6 Polar Or Non Polar Quora

Trigonometry Functions Exponentials On The Clep Calculator Video Lesson Transcript Study Com

Solution 34507 Performing Polar And Rectangular Conversions On The Ti 83 Plus And Ti 84 Plus Family Of Graphing Calculators
.png)
Peptide Calculator Biosynth Biosynth

Gas Chromatographic Estimation Of Vapor Pressures And Octanol Air Partition Coefficients Of Semivolatile Organic Compounds Of Emerging Concern Journal Of Chemical Engineering Data
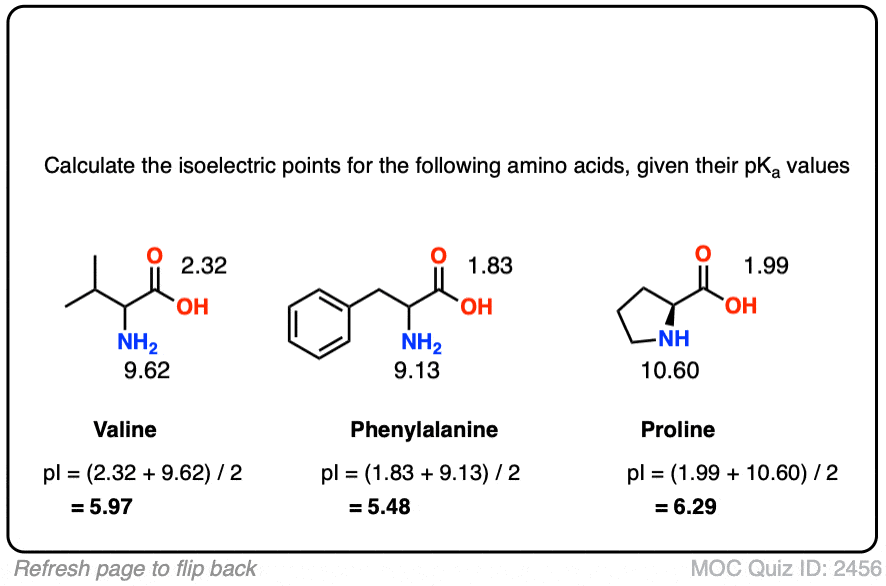
Isoelectric Points Of Amino Acids And How To Calculate Them Master Organic Chemistry

Polarity Meaning Definition Example Polar Vs Non Polar Molecules Wiith Faqs And Videos On Polarity
Percent Ionic Character Calculator
How To Calculate The Dipole Moment Of Water Quora
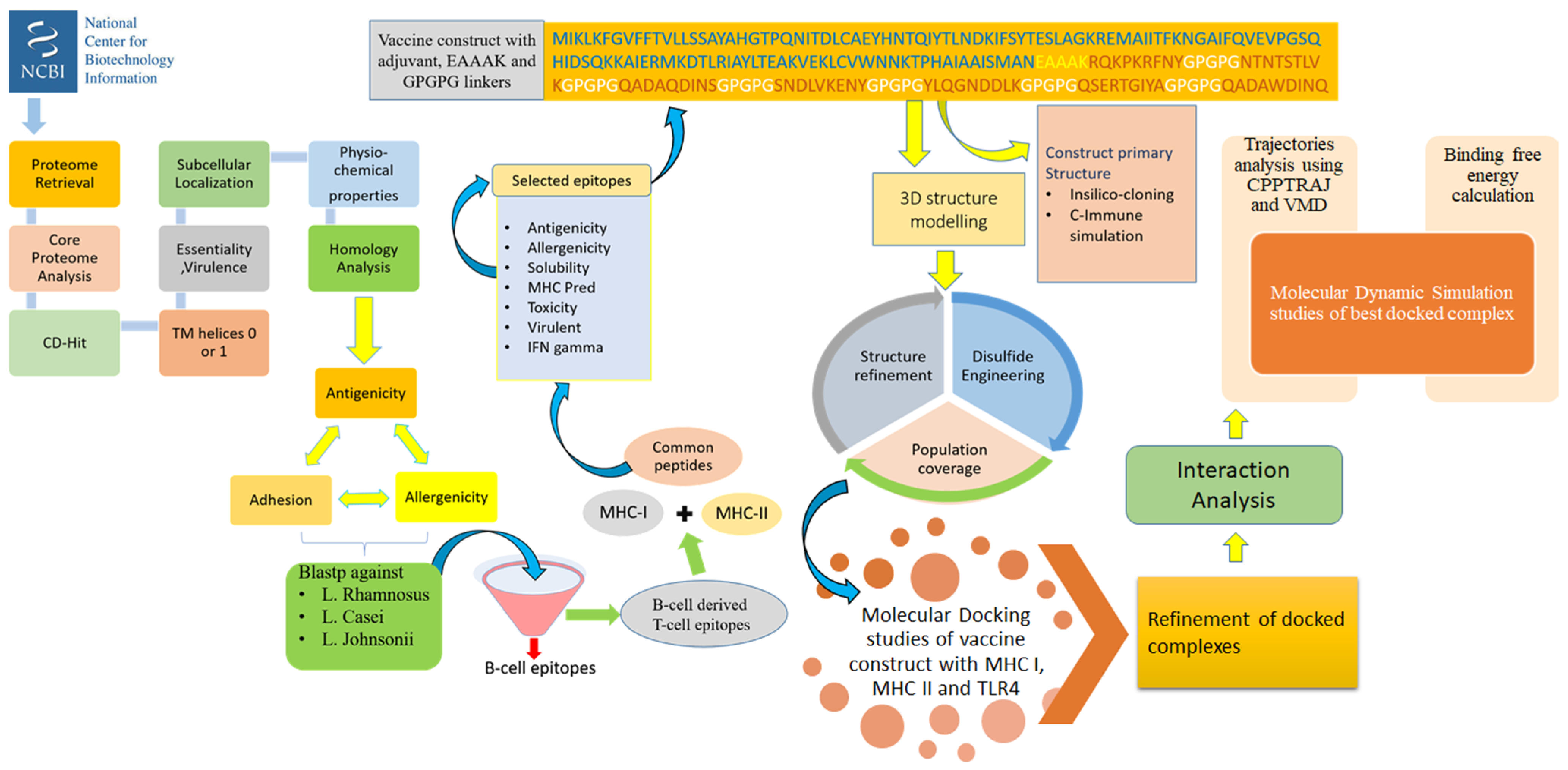
Vaccines Free Full Text A Comprehensive Computer Aided Vaccine Design Approach To Propose A Multi Epitopes Subunit Vaccine Against Genus Klebsiella Using Pan Genomics Reverse Vaccinology And Biophysical Techniques

4 Ways To Calculate Electronegativity Wikihow

Scn Formal Charge How To Calculate It With Images Covalent Bonding Chemical Bond Interesting Information
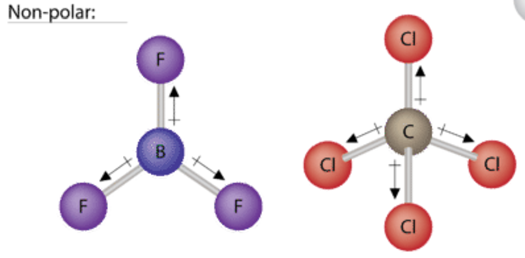
4 12 Shapes And Properties Polar And Nonpolar Molecules Chemistry Libretexts
![]()
Peptide Amino Acids Calculator Bachem

Equilibrium Partition Coefficients Of Diverse Polar And Nonpolar Organic Compounds To Polyoxymethylene Pom Passive Sampling Devices Environmental Science Technology